Estimated reading time: 6 minutes
As discussed in part I, the hype of using ivermectin to treat COVID-19 has been heavily based on unreliable or manipulated data.
These have led to the spread of ivermectin misinformation and the retraction of the supporting scientific studies.
But what does reliable science tells us about using Ivermectin to combat COVID-19?
Let’s go over four scientific studies published in renowned journals and find out!
Side Note. Impact factor (IF) is the metric scientists use to assess how important a journal is to its field. This metric reflects the yearly mean number of citations of articles published in the last two years in a journal.
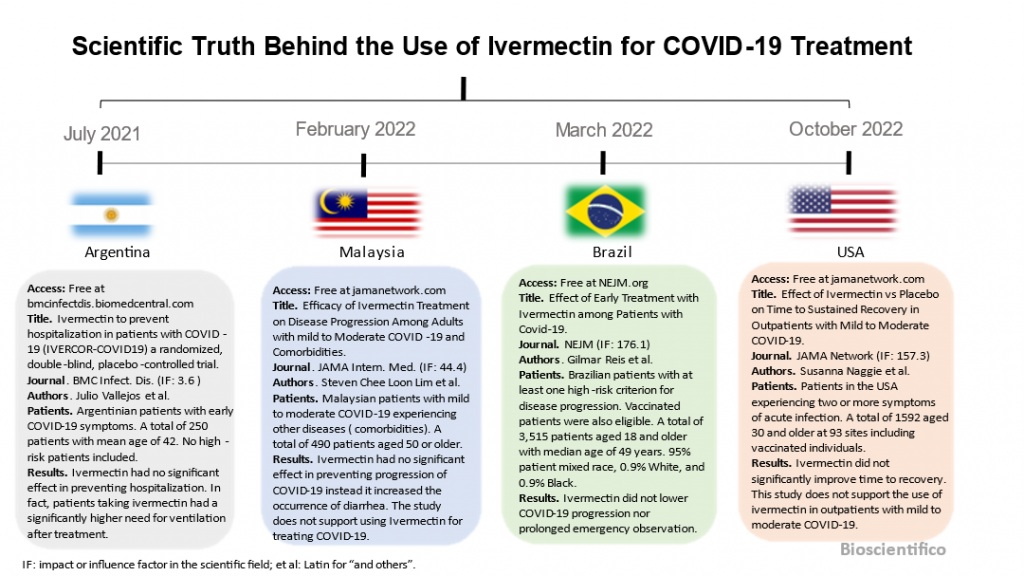
Ivermectin study with Argentinian patients (#patients=250)
Julio Vallejos and colleagues published this study in BMC infectious disease (IF: 3.6) on July 2021 (See Figure 1).
Their objective was to find whether Ivermectin treatment can prevent hospitalization in patients with early COVID-19.
To answer this, they performed a randomized, double-blind, placebo-controlled study in non-hospitalized people with COVID-19 in Corrientes, Argentina.
Randomized means exactly that, that patients were organized into groups randomly without bias. Double-blind means that both the researcher and patient are blind to what treatment which patient is receiving. Placebo-controlled means that there is treatment with the actual drug and a control (aka harmless pill with no therapeutic effect).
People with positive nasal swabs were contacted within 48 hours to participate as the researchers focused on early COVID-19 patients. A total of 250 patients were randomized to Ivermectin or placebo group.
The main thing scientists were looking for was the effectiveness of Ivermectin in preventing early COVID-19 patient hospitalization.
What do their results show?
Results showed that patient hospitalization in the Ivermectin group (5.6%) was similar to patients taking placebo(8.4%). Doing statistical analysis, these results hada p-value of 0.227.
The p-value is an extremely fundamental statistical measure as it allows researchers to know whether the results have any significance. In other words, it will tell us the probability of obtaining the same observed results, giving a measure of credibility.
A p-value of less than 0.05 is considered of significant importance. Therefore, the p-value of 0.227 in this study indicates no significant effect of Ivermectin in preventing hospitalization.
Interestingly, it was also observed that the average time to invasive mechanical ventilatory support was shorter ( 5 days) for patients taking Ivermectin compared to patients taking the placebo (10 days). These data yielded a p-value of 0.019.
These results indicate that Ivermectin significantly increased the time for early COVID-19 patients to need ventilation support. In conclusion, the researchers found that Ivermectin had no significant effect in preventing hospitalization.
Patients taking Ivermectin needed ventilation support sooner reflecting a more rapid disease progression.
Importantly, the data from this study were also used to correct statistical analyses done in another scientific paper.
Study with Malaysian patients (#patients= 490)
Steven Chee Loon Lim and colleagues published this study in the Journal of the American Medical Association Internal Medicine (IF:44.4) on February 2022.
They asked if adding Ivermectin to the standard of care reduced risk of severe disease.
To answer this, the investigators performed an open-label randomized clinical trial of patients with a high risk of COVID-19 progression. Open-label is the opposite of double-blind studies, where both the researchers and participants know of the drug or treatment given.
The study involved 490 patients 50 years and older with lab-confirmed COVID-19, comorbidities, and mild to moderate disease.
Patients were randomized to receive either oral Ivermectin with the standard of care or just the standard of care alone. Here standard of care meant symptomatic therapy and continuous monitoring of the disease
What can we learn from their results?
The main outcome the researchers analyzed was which patient population progressed faster to severe disease.
This was defined as needing supplemental oxygen as patients go into a stage with low oxygen levels (hypoxic).
Results showed patients in the Ivermectin group progressed to severe disease similarly to those not taking Ivermectin with a statistical value of p=0.25.
You guessed it, not significant. The most common side effect was diarrhea occurring more in patients taking Ivermectin (5.8%) than in the control group (1.6%).
In conclusion, this study does not support using Ivermectin for patients with COVID-19.
Study with Brazilian patients (#patients= 3,515)
As I further discuss, the Brazilian government has been a key player in promoting Ivermectin as a drug for COVID-19.
Gilmar Reis and colleagues published a study in an even more influential journal, The New England Journal of Medicine( NEJM, IF: 176.1)), in March 2022.
The researchers asked whether Ivermectin could be used to prevent hospitalization or extended treatment for COVID-19.
To answer this, they performed a double-blind, randomized, placebo-controlled, adaptive platform trial involving 3,515 symptomatic patients from Brazil.
Adaptive platform simply means a clinical trial that is adapted to study multiple treatments or disease interventions continuously.
The main outcome analyzed was hospitalization or an emergency department visit due to COVID-19 worsening.
What do their results show?
Overall, results showed that 14.7% of patients taking Ivermectin compared to 16.3% taking placebo had a primary outcome group. Again, no significant effects of Ivermectin use were found. In conclusion, treatment with Ivermectin did not result in a lower rate of medical admission.
Study with US patients (#patients= 1,591)
Many political figures and influencers from the US have promoted using Ivermectin without any scientific backup. This has been likely influenced by political agenda and monetary reward.
The last study I want to discuss is the one using a US patient cohort published in JAMA network (IF:157.3) by Susanna Naggie and colleagues in October 2022.
Researchers asked whether ivermectin can shorten symptom duration or prevent hospitalization in people with mild to moderate symptomatic COVID-19.
To answer this, they performed a decentralized, double-blind, randomized, placebo-controlled trial with a total of 1591 patients aged 30 years and older with confirmed COVID-19 at 93 sites in the US.
Decentralized means that researchers used telemedicine and mobile/local healthcare providers as defined by the Food and Drug Administration (FDA).
The main thing researchers were measuring was the time for recovery, where recovery meant three consecutive days without any symptoms. Among patients, 47% reported receiving at least two doses of a COVID-19 vaccine.
Let the data speak
Results showed that the median time of recovery was 12 days in the ivermectin group compared to 13 days in the placebo group.
There were also 10 hospitalizations in the ivermectin and 9 in the placebo group.
In conclusion, ivermectin treatment did not significantly improve time to recovery. This study does not support the use of ivermectin in patients with mild to moderate COVID-19.
In summary, these studies, which have been reviewed by other scientists in the field, hence the term “peer-reviewed”, indicate that ivermectin does not help at all as a drug against COVID-19 progression.
On the contrary, it increased the need for ventilation and the secondary effect of diarrhea as seen in some of these studies.
But if the COVID-19 patient is not benefiting from Ivermectin, who is? And what can we learn from this ivermectin craze? I discuss these in my last blog of the ivermectin series, “ Ivermectin for COVID-19: the take-home message”
- Five factors behind Latin America’s Alarming Dengue Spike
- How H. pylori does not discriminate against Hispanics/Latinos
- What’s your Latino background? You could be infected with Helicobacter Pylori
- What Hispanics think of using AI in healthcare and medicine
- A COVID-19 political remedy to worry about

